Vapor Pressure Calculator
With this vapor pressure calculator, we present to you two vapor pressure equations! Have you found yourself wondering: what is vapor pressure? How does a liquid change into a gas due to a change in pressure and temperature? When and how do I use the Clausius-Clapeyron equation? And what does this all have to do with the enthalpy of vaporization? If you have — keep reading. We will answer all of these questions and more!
What is vapor pressure?
Vapor pressure is the pressure exerted by the vapor molecules of a substance in a closed system. It occurs at equilibrium, i.e., when the molecules are both vaporizing and condensing at the same rate at a particular pressure. Vapor pressure is directly related to the kinetic energy of a molecule. Light molecules, those with high kinetic energy or those with weak intermolecular forces, have higher vapor pressures and, therefore, a higher volatility — the tendency to vaporize.
How to calculate vapor pressure? Let's have a closer look at two vapor pressure equations: the Clausius-Clapeyron equation and Raoult's law.
Clausius-Clapeyron equation
The Clapeyron equation states:
where:
- — Derivative of pressure with respect to temperature;
- — Specific latent heat — the thermal energy absorbed or released during a phase transition;
- – Temperature; and
- — Change of the specific volume during a phase transition.
This formula is illustrated in a phase diagram — a chart showing how phases change and coexist at equilibrium at different pressures and temperatures.
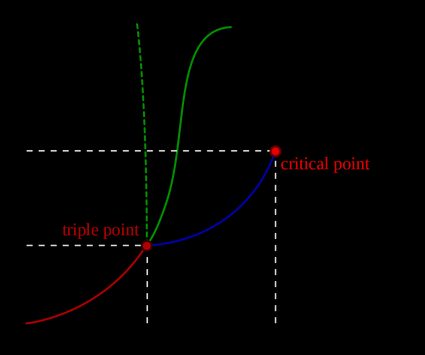
The Clausius-Clapeyron equation is a derivation of this formula. It describes the relationship between the vapor pressure and the temperature of a liquid. It's accurate for the phase transition between liquid and gas (vaporization) or solid and gas (sublimation). When there is a big difference between the specific volume of a molecule's gas phase and its condensed phase, we can derive the following equation:
where:
- — Initial temperature measured in Kelvin (K);
- — Final temperature (K);
- — Initial pressure;
- — Final pressure;
- — Molar enthalpy of vaporization or sublimation (); and
- — Gas constant of .
While solving problems in physics and chemistry, it's important to remember to convert your units. Don't worry about it here — our vapor pressure calculator will convert them for you. But if you're solving the Clausius-Clapeyron equation on your own, remember that temperature should always be expressed in Kelvins. The unit of pressure doesn't matter as long as it's the same for both initial and final pressure.
What is the enthalpy of vaporization?
Enthalpy of vaporization or, in other words, the heat of vaporization, is the energy required for a phase change — turning a liquid into a gas. Analogously, the amount of energy needed for a direct phase transition between a solid and gaseous state is called the enthalpy of sublimation.
Clausius-Clapeyron equation — an example
In your chemistry classes, a teacher might give you an exercise to find the vapor pressure or enthalpy of vaporization. Let's solve one to help you fully understand the Clausius-Clapeyron equation.
- Water has an enthalpy of vaporization of 40660 J/mol (ΔHvap).
- Its vapor pressure is 102325 Pa (P1) at a temperature of 280 K (T1).
- What is its pressure at 263 K (T2)?
Let's start with calculating the right side of our equation, as there are no unknowns:
By multiplying both sides by the exponent, we get:
If you aren't familiar with natural logarithms () or the exponential growth constant (), we highly recommend our logarithm calculator. You can rearrange the above equation to solve for :
As you see, it's a bit complicated to do this calculation by hand. It's much easier to use a scientific calculator or, as long as you are here, our vapor pressure calculator :).
How to calculate vapor pressure? — Raoult's law
Raoult's law states that:
The vapor pressure of a solution is equal to the vapor pressure of a pure solvent times its mole fraction.
It's also expressed by the following equation:
where:
- — Vapor pressure of a solution;
- — Vapor pressure of the solvent;
- — Mole fraction of the solvent — the ratio of moles of the solvent to moles of the entire solution.
If there are more than two components in the solution, Dalton's law of partial pressures must be applied.
Raoult's law is only accurate for ideal solutions. A solution is considered ideal when the interactions between all molecules are the same as the interactions between molecules of the same chemical. Mixtures of different molecules are never ideal, but we can treat them as if they were to simplify our calculations.
Let's use this vapor pressure equation in an exercise:
What is the vapor pressure of a solution made by dissolving 100 grams of glucose (C6H12O6) in 500 grams of water? The vapor pressure of pure water is 47.1 torr at 37 °C.
- Calculate the mole fraction of water (the solvent).
-
-
Molar mass of water is 18 g/mol, and for glucose it is 180.2 g/mol.
-
-
-
-
- Using Rault's law:
Now you know how to calculate vapor pressure on your own. Both of these equations might look intimidating at first, but once you understand them, they turn out to be very easy. Remember to check out our other calculators, for example, the osmotic pressure calculator.
What is the boiling temperature at 60% of the atmospheric pressure (0.6 atm)?
It is 86.35 °C. You can use the Omnicalculator Vapor pressure calculator or the Clausius Claperyron equation as follows:
-
Define your first point. For example, water boils at 100 °C when pressure is 1 atm.
-
Obtain the water enthalpy of vaporization: 40 660 J/mol. Also, remember we are going to use the gas constant: 8.3145 J/mol·K
-
Resolve the vapor pressure equation considering the 2nd point pressure is 0.6 atm. You will get the resulting temperature: 86.35 °C.
How vapor pressure affects your house pump?
As per the Clausius Clapeyron equation, a lower vapor pressure corresponds to a lower boiling point. That means that when your house pump sucks water by reducing pressure at the inlet point, it is more prone to evaporate. If this is not controlled, we get cavitation inside the pump, which is violent water vaporization followed by explosive condensation that can damage pump internals.
How to calculate boiling point knowing vapor pressure?
We can use the Omnicalculator tool Vapor pressure calculator or the Clausius Clapeyron equation as follows:
- Define a boiling temperature and pressure you know. Let's say 100 °C, at 101.3 kPa of atmospheric pressure.
- Find out the new pressure at which water will boil. Hint, air pressure at 3500 m.a.s.l., at 20 °C is 65 kPa.
- By using the vapor pressure equation, we get that water would boil at 88.12 °C.
How does vapor pressure affect boiling point?
As per the Clausius Clapeyron equation, a lower vapor pressure corresponds to a lower boiling point temperature Note that boiling occurs when water pressure is equal to atmospheric pressure. Consequently, if we reduce environmental pressure as it happens in reality at higher altitudes, we get a lower boiling point.